Volume 16, Number 7—July 2010
Dispatch
Detection of Lassa Virus, Mali
Abstract
To determine whether Lassa virus was circulating in southern Mali, we tested samples from small mammals from 3 villages, including Soromba, where in 2009 a British citizen probably contracted a lethal Lassa virus infection. We report the isolation and genetic characterization of Lassa virus from an area previously unknown for Lassa fever.
Lassa fever is an acute viral infection associated with a wide spectrum of disease manifestations, which range from mild to hemorrhagic fever characterized by multiorgan failure (1). The etiologic agent of Lassa fever is Lassa virus (LASV, family Arenaviridae, genus Arenavirus), which is maintained in its natural rodent reservoir, the multimammate rat (Mastomys natalensis) (1–3). Although M. natalensis rats are ubiquitous in many parts of sub-Saharan Africa, infected rodents have only been reported in West African countries, most commonly Nigeria, Sierra Leone, and Guinea (3–5). Consequently, cases of Lassa fever mainly occur in regions in which the virus is endemic, consisting of those 3 countries and Liberia, with an annual incidence ranging from 300,000 to 500,000 cases and ≈5,000 deaths (6). Serologic evidence of LASV infections has also been reported from other West and Central African countries (5,7–9). Additionally, LASV has been introduced into Europe and North America several times, making Lassa fever one of the most prominent imported exotic viral hemorrhagic fevers, which strongly affects public health systems (9).
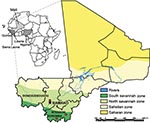
In 2009, Lassa fever was diagnosed postmortem in a young man with a 10-day history of fever who had been evacuated from Mali to London (10). The patient had no travel history to any LASV-endemic region, which suggests that he contracted the infection in Mali, most likely while working in the village of Soromba (Figure 1). In this report, we provide evidence that LASV is circulating in M. natalensis in southern Mali, thereby expanding the geographic distribution of LASV in West Africa and posing a risk to humans in an area previously unknown for Lassa fever. The study was carried out in accordance with a protocol approved by an Institutional Animal Care and Use Committee of the US National Institutes of Health.
From June 5 through 14, 2009, a total of 103 small mammals (trap success rate of 17.6%) were live trapped and sampled from 3 villages in Mali (Figure 1; Table): N’Tessoni (11°2′0′′N, 5°59′0′′W), Soromba (10°35′0′′N, 7°9′0′W), and Doneguebougou (12°48′21′′N, 7°59′0′′W). Pertinent information was recorded for each animal, and ear punch, heart, blood, lung, and liver samples were collected. Mastomys sp. Rats were the predominant rodent captured (82/103, 79.6%), with all but 4 of these animals identified as M. natalensis rat by cytochrome B sequence analysis of DNA isolated from ear punch specimens (11; GenBank accession nos. HM130517–HM130519).
Total RNA was extracted from tissue specimens and blood by using RNeasy or QIAamp viral RNA kits (QIAGEN, Valencia, CA, USA), respectively, and screened for the presence of LASV RNA by using a SYBR-green based, real-time reverse transcription–PCR assay that amplifies a 195-bp portion of the small genomic segment (primers Gc656s: 5′-ATTGCTCTTGACTCAGGCC-3′ and Gc851as: 5′-GTGTCCATGTGAATGTGCCTA-3′; TIB Molbiol GBH, Adelphia, NJ, USA). Six infected rodents were identified, each of which had LASV-positive lung, liver, and blood specimens. Positive rodents were genetically identified as M. natalensis, and all were captured from Soromba, for a village prevalence of 24% (6/25; Table). Four (66.7%) of 6 infected rodents were male and 5 (83.3%) of 6 were adult. All 6 infected animals were captured indoors.
Tissue homogenates were prepared from selected LASV-positive animals and passaged twice on subconfluent monolayers of Vero E6 cells. After 2 passages, no discernible cytopathic effect was observed, although LASV RNA was detected by reverse transcription–PCR in cells and supernatant. Virus isolation was confirmed by immunoblot analysis in infected cells and supernatant by using a LASV nucleoprotein-specific monoclonal antibody, which detected a 50–55-kDa protein consistent in size with the LASV nucleoprotein ( 12; Appendix Figure). Furthermore, electron microscopy performed on infected cells showed viral particles consistent in size and morphologic features with an arenavirus (Appendix Figure, panel B).
An ≈800-nt fragment of the LASV polymerase gene was amplified from the isolated virus and the tissues of infected rodents by using a pan–Old World arenavirus assay (13). Additionally, an 873-bp fragment of the LASV glycoprotein gene, corresponding to the sequence from a British Lassa fever patient (10), was amplified from the same samples by using primers Gc F1 5′-GCATTTTAATTCAGCCTCAATTAAC-3′ and Gc R1 5′-ATGGGGCAGATTGTGACATTCTTTC-3′. Amplicons were sequenced (GenBank accession nos. GU573541–GU573546 and GU573547–GU573552 for polymerase and glycoprotein fragments, respectively) and aligned with previously described arenavirus sequences by using ClustalX version 2.0.10 software (www.clustal.org). Nucleotide sequences from the isolated LASV were indistinguishable from the sequences generated from the infected rodent tissues from which they were derived (data not shown). Phylogenetic analysis of the glycoprotein sequences confirmed that all rodent-derived LASV sequences belonged to the same genetic clade as the sequence of the imported Lassa fever case in the United Kingdom. Polymerase fragment sequences confirmed that this clade is most closely related to the previously described AV strain of LASV that originated from the neighboring countries of Côte d’Ivoire, Burkina Faso, or Ghana (14; Figure 2).
This study demonstrates that M. natalensis rats in Mali carry a genetically unique strain of LASV (proposed name Soromba-R). Genetically, Soromba-R is nearly indistinguishable from the nucleotide sequence generated from a lethal case of Lassa fever imported from Mali to the United Kingdom, which supports the epidemiologic data that the infection occurred in the village of Soromba in southern Mali (10). The Soromba-R strain of LASV is genetically closely related to the AV strain, which was isolated from an imported lethal Lassa fever case in a patient who contracted the infection while traveling in neighboring countries (14). Thus, this study provides evidence for an expanded region of LASV endemicity in West Africa.
On the basis of Mali’s geographic proximity to countries where LASV is endemic, it should not be surprising that LASV is circulating there. Cases of Lassa fever are diagnosed annually in Guinea, and sporadic cases have been diagnosed in Burkina Faso and Côte d’Ivoire, all of which border Mali (5,14,15). Furthermore, although to date only 1 laboratory-confirmed Lassa fever case has been diagnosed from Mali, there is serologic evidence of a LASV infection in 1971 in a missionary stationed in Mali (8). Considering this probable case and the identification of a genetically unique strain of LASV in rodents and a human case from Mali, it is likely that LASV has been present in Mali yet undetected for decades. The nonspecific clinical signs and symptoms of a large proportion of LASV infections, combined with the unfamiliarity of Malian physicians with Lassa fever, suggest that the infection could easily be misdiagnosed. Currently, Lassa fever is not considered in the differential diagnosis for febrile illness in Mali. This report, however, provides evidence of an emerging infectious disease problem with public health effects; thus, Lassa fever diagnostics and surveillance should be implemented at least in the southern regions of Mali.
Soromba and the surrounding area may represent an enzootic hotspot for LASV-infected rodents. However, a greater distribution of LASV in Mali should not be ruled out, particularly in the southern regions of the country where the climate and geography are similar to conditions found in many parts of Guinea and Sierra Leone (Figure 1). Combined with our findings that M. natalensis was the predominant species of rodent captured in all 3 villages in Mali suggests that conditions favor LASV circulation. Furthermore, on the basis of the timing of the peak incidence of Lassa fever cases, which typically corresponds with the dry season (15), we believe that the proportion of LASV-infected rodents may have been low at the time of these studies and beyond the sensitivity of the limited sampling effort conducted here. Future studies need to address these concerns as well as determine the geographic distribution of LASV-infected rodents in Mali to help focus public health preparedness efforts.
Dr Safronetz is a visiting fellow in the Disease Modeling and Transmission Section of the Laboratory of Virology at the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. His research interests include the ecology, epidemiology, and pathogenesis of rodent-borne viruses, including hantaviruses and arenaviruses.
Acknowledgments
We thank the village elders and leaders who allowed us to collect rodents from their communities. These studies would not have been possible without logistical support in Mali from Richard Sakai and assistance in the field from Ousmane Maiga, Moussa Keïta, Yoro Sidibe’, and Moro Diakite. We also thank Anita Mora and Dansine Diarra for assisting in preparing the figures and Kent Barbian and Amanda Favreau for performing the sequencing reactions.
This research was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. All laboratory work with potentially infectious materials was conducted in a Biosafety Level 4 facility at the Rocky Mountain Laboratories.
References
- Buchmeier MJ, de la Torre JC, Peters CJ. Arenaviridae: the viruses and their replication. In: Knipe DL, Howley PM, editors. Fields Virology, 5th edition. Philadelphia: Lippincott-Raven; 2007. p. 1791–828.
- Frame JD, Baldwin JM Jr, Gocke DJ, Troup JM. Lassa fever, a new virus disease of man from West Africa: clinical description and pathological findings. Am J Trop Med Hyg. 1970;19:670–6.PubMedGoogle Scholar
- Monath TP, Newhouse VF, Kemp GE, Setzer HW, Cacciapuoti A. Lassa virus isolation from Mastomys natalensis rodents during an epidemic of Lassa fever in Sierra Leone. Science. 1974;185:263–5. DOIPubMedGoogle Scholar
- Granjon L, Duplantier JM, Catalan J, Britton-Davidian J. Systematics of the genus Mastomys (Thomas, 1915) (Rodentia: Muridae): a review. Belg J Zool. 1997;127:7–18.
- Fichet-Calvet E, Rogers DJ. Risk map of Lassa fever in West Africa. PLoS Negl Trop Dis. 2009;3:e388. DOIPubMedGoogle Scholar
- Ogbu O, Ajuluchukwu E, Uneke CJ. Lassa fever in West Africa sub-region: an overview. J Vector Borne Dis. 2007;44:1–11.PubMedGoogle Scholar
- World Health Organization. WHO Lassa fever fact sheet no. 179. Geneva: The Organization; 2000.
- Frame JD. Surveillance of Lassa fever in missionaries stationed in West Africa. Bull World Health Organ. 1975;52:593–8.PubMedGoogle Scholar
- Macher AM, Wolfe MS. Historical Lassa fever reports and 30-year clinical update. Emerg Infect Dis. 2006;12:835–7.PubMedGoogle Scholar
- Atkin S, Anaraki S, Gothard P, Walsh A, Brown D, Gopal R, The first case of Lassa fever imported from Mali to the United Kingdom, February 2009. Euro Surveill. 2009;14:1–3.PubMedGoogle Scholar
- Lecompte E, Granjon L, Peterhans JK, Denys C. Cytochrome b–based phylogeny of the Praomys group (Rodentia, Murinae): a new African radiation? C R Biol. 2002;325:827–40. DOIPubMedGoogle Scholar
- Branco LM, Matschiner A, Fair JN, Goba A, Sampey DB, Ferro PJ, Bacterial-based systems for expression and purification of recombinant Lassa virus proteins of immunological relevance. Virol J. 2008;5:74. DOIPubMedGoogle Scholar
- Vieth S, Drosten C, Lenz O, Vincent M, Omilabu S, Hass M, RT-PCR for detection of Lassa virus and related Old World arenaviruses targeting the L gene. Trans R Soc Trop Med Hyg. 2007;101:1253–64. DOIPubMedGoogle Scholar
- Gunther S, Emmerich P, Laue T, Kuhle O, Asper M, Jung A, Imported Lassa fever in Germany: molecular characterization of a new Lassa virus strain. Emerg Infect Dis. 2000;6:466–76. DOIPubMedGoogle Scholar
- Bausch DG, Demby AH, Coulibaly M, Kanu J, Goba A, Bah A, Lassa fever in Guinea: I. Epidemiology of human disease and clinical observations. Vector Borne Zoonotic Dis. 2001;1:269–81. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 16, Number 7—July 2010
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Heinz Feldmann, Rocky Mountain Laboratories, National Institutes of Health, 903 S 4th St, Hamilton, MT 59840, USA
Top